How Ammonia is Made in Bulk: Inside the Haber-Bosch Process
Ever wondered how ammonia is made in bulk to meet the needs of agriculture, industry, and even medicine? The answer lies in a groundbreaking method called the Haber-Bosch process—a century-old innovation that changed the world. Whether you’re a student, curious mind, or a non-chemist, this post will guide you through the science and scale behind one of the most important chemical reactions in human history.
Why Is Ammonia So Important?
Before diving into how ammonia is made in bulk, let’s understand why it matters.
Ammonia (NH₃) is a colorless gas with a pungent smell, but don’t let that fool you—it’s vital for life. It’s used in:
- Fertilizers (over 80% of global ammonia production!)
- Cleaning agents
- Refrigeration systems
- Pharmaceuticals
- Textile and plastics manufacturing
Without industrial ammonia, global agriculture would struggle to feed billions. That’s why the Haber-Bosch process is often called one of the most important scientific discoveries of the 20th century.
What Is the Haber-Bosch Process?
Developed by Fritz Haber (chemist) and Carl Bosch (engineer) in the early 1900s, this process allows industries to synthesize ammonia on a massive scale using two simple ingredients:
- Nitrogen (N₂) from the air
- Hydrogen (H₂) usually from natural gas or water
The chemical equation is:
N₂ + 3H₂ → 2NH₃
(Under high temperature and pressure, with an iron catalyst)
It’s simple on paper but highly complex in execution. Here’s why.
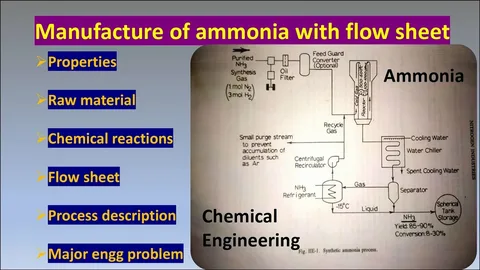
Step-by-Step: How Ammonia Is Made in Bulk
Let’s break it down into digestible stages.🔹 1. Nitrogen Extraction
Air is made up of about 78% nitrogen. To extract it:
- Air is compressed and cooled
- Oxygen is separated via distillation, leaving pure nitrogen
This nitrogen is one of the key reactants in the Haber-Bosch process.
2. Hydrogen Generation
Hydrogen can be made in several ways, but the most common is steam reforming of natural gas:
- Natural gas (mainly methane) reacts with steam
- Produces hydrogen gas and carbon dioxide
This stage also has an environmental cost, which we’ll explore later.
3. Compression & Mixing
The purified nitrogen and hydrogen gases are:
- Mixed in a 1:3 ratio
- Compressed to around 150–300 atmospheres
4. Reaction Chamber
This is the heart of the process. Inside a large reaction vessel:
- The gas mixture is heated to about 400–500°C
- Passed over an iron catalyst
Here, the gases react to form ammonia. But not all the gas turns into ammonia in one go, so…
5. Cooling and Separation
The gas mix is cooled:
- Ammonia condenses into liquid
- Unused nitrogen and hydrogen are recycled and sent back into the system
This loop makes the process more efficient and cost-effective.
A Layman Analogy: Cooking Under Pressure
Think of it like making a stew:
- You add ingredients (nitrogen and hydrogen)
- Use a pressure cooker (reaction chamber)
- Add a seasoning (iron catalyst)
- Turn up the heat (400°C+)
- Some ingredients become stew (ammonia), and leftovers go back into the pot for another round
Simple enough?
Global Impact of the Haber-Bosch Process
This isn’t just chemistry—it’s civilization-altering innovation.
Agriculture
Thanks to synthetic ammonia, fertilizers are affordable and accessible. That means:
- Increased crop yields
- Reduced global hunger
- Sustainable farming for a growing populationAccording to Science.org, nearly half of the world’s population is supported by food grown using synthetic nitrogen fertilizers.
Industrial Use
Aside from fertilizers, ammonia is used in:
- Plastics
- Dyes
- Cleaning agents
- Pharmaceuticals
It’s even used in the production of explosives, which played a major role in both world wars (though that’s a story for another day).
Environmental Concerns and Green Alternatives
The process, while revolutionary, isn’t perfect.
- High energy use: The reaction needs extreme heat and pressure.
- Carbon footprint: Hydrogen production from fossil fuels releases CO₂.
Emerging Solutions
- Green ammonia: Using renewable hydrogen from water electrolysis
- Catalyst innovation: Research into better catalysts at lower temperatures
- Modular systems: Small-scale, localized ammonia plants for rural areas
For those interested, Nature.com dives deep into innovations around green ammonia production.
Quick Recap Table
| Stage | Description |
|---|---|
| Nitrogen Extraction | From air via liquefaction and distillation |
| Hydrogen Generation | From natural gas (steam reforming) |
| Gas Mixing | Nitrogen and hydrogen mixed 1:3 |
| Compression | Up to 300 atmospheres |
| Reaction | Iron catalyst + heat (400–500°C) |
| Separation | Ammonia cooled and collected, rest recycled |
Conclusion
Now you know how ammonia is made in bulk—a process that powers farms, factories, and more. The Haber-Bosch process is not just a chemical reaction; it’s the beating heart of modern civilization.
While it’s not without its flaws, continuous innovation is helping to make it more sustainable. Understanding this process is key, not just for chemists, but for anyone who wants to grasp the science behind the food on our plates and the products we use daily.
❓ FAQs: How Ammonia is Made in Bulk
1. What is the main raw material for ammonia production?
Nitrogen from the air and hydrogen—usually from natural gas—are the two main ingredients.
2. Why is high pressure needed in the Haber-Bosch process?
High pressure favors the formation of ammonia by shifting the equilibrium of the reaction towards the product.
3. Is ammonia production harmful to the environment?
Yes, it has a significant carbon footprint due to hydrogen production, but green alternatives are being developed.
4. What catalyst is used in ammonia production?
Iron-based catalysts are most commonly used to speed up the reaction.
5. Can ammonia be produced sustainably?
Yes, through green ammonia using renewable hydrogen, though it’s still in early adoption stages.